


The tighter the nucleus holds an electron, the more energy needed to remove it. Ionization Energy is the energy needed to remove an electron from a gaseous atom.
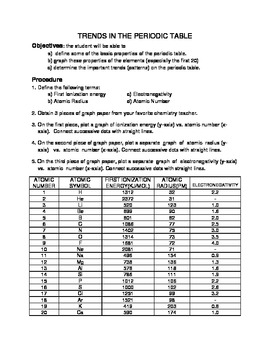
Atoms with low electronegativity are highly electropositive. Think of it as the opposite of electronegativity. electronegativity decreases as you move down the tableĪ related term is electropositive.electronegativity increases as you move left to right across the table.In general, the higher the electronegativity, the stronger the force of attraction between bonded electron and the atom nucleus. Both of these reduce the force of attraction between the nucleus and the added electron.Įlectronegativity is the measure of attraction between the atom’s nucleus and electrons in a chemical bond. As you move down, the outer electron is both shielded from the nucleus by filled shells and physically further away. electron affinity decreases as you move down (with exceptions)Ītoms with stronger nuclear charge tend to have higher electron affinities as you move across the table.electron affinity increases as you move left to right.It is measured by the energy change in the atom as an electron is added to the gaseous form of the atom. The outer electrons are not held as tightly as the outer electrons of the elements above them on the table.Įlectron Affinity is the ability of an atom to accept an electron. These outer shells are shielded from the positive charge of the nucleus by layers of electrons and the effective charge they experience is less than the element above them. As you move down the periodic table, the valence of the the atom remains the same, but there are more filled electron shells between the outer electrons and the positive nucleus. The electrons form shells and are attracted strongly to the positive charge in the nucleus, pulling the shells closer to the center and effectively making the atom smaller with the addition of each proton. atomic radius increases as you move downĪs you move across the periodic table from right to left, each element contains one more electron and one more proton.atomic radius increases as you move right to left.The organization of the periodic table shows the periodic trends of six different physical properties of the elements: atomic radius, electron affinity, electronegativity, ionization energy, and metallic/nonmetallic character.Ītomic radius is half the distance between two identical atoms touching each other.


 0 kommentar(er)
0 kommentar(er)
